Tungsten Oxide Thin Film Electrode Oxidation Glucose
Glucose exists in the nature by photosynthesis. Due to its abundant volume, low cost and reproducible, it is regarded as the main energy substrates to produce hydrogen. Glucose is the main waste of agriculture, food and paper-making industry, improper disposition will cause damage to environment. Recently many PEC systems produce hydrogen by glucose.
Tungsten oxide connects with electrocatalyst to produce hydrogen from glucose shows good photocatalytic activity, deposit electrocatalyst on the surface of photocatalyst can promote photocatalytic activity of semiconductor. Electrocatalyst deposited on the surface of semiconductor will form a layer of cover. By changing electron distribution in the system, the surface property of WO3 will be affected, so the photocatalytic activity is improved. Usually if Fermi level of WO3 is higher than the two combined material, electron will keep migrating from WO3 to depositing electrocatalyst. The shallow well potential Schottk energy barrier which can trap electron will form on the surface of metal and electrocatalyst. It provides effective trap potential for separating of photo electron and electron hole, it can resist the composite of photo electron and electron hole further, also the separating efficiency of charge carrier, thus to improve the quantum efficiency of photocatalyst.
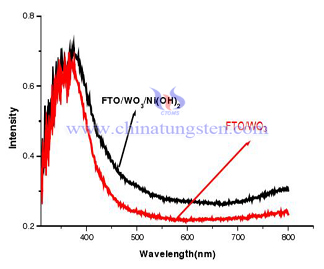
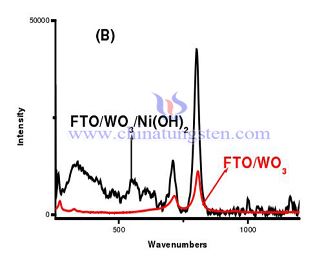
Use FTO/WO3/Ni(OH)2 thin film electrode in reduction of glucose experiment. Through this experiment, we can find that exposure of WO3 thin film electrode without Ni(OH)2 barely have photoelectrocatalytic glucose effect. Depositing Ni(OH)2 on the surface of tungsten oxide thin film can enhance photoelectric effect. Below is the raman spectrum and ultraviolet visible light absorption curve comparison of FTO/WO3 thin film electrode and FTO/WO3/Ni(OH)2.
If you have any inquiry of tungsten oxide, please feel free to contact us:
Tel.: +86 592 5129696 +86 592 5129595
Fax: +86 592 5129797



评论
发表评论