Hexagonal Tungsten Trioxide
Tungsten trioxide is a unique n-type semiconductor material, that makes it one of the few oxide semiconductors which are easy-to-realize quantum size effect, and performances the excellent properties in photocatalysis, electrochromic, photochromic, gasochromic and other aspects, thus widely used in fields of chemical sensor, fuel cell, light catalyst and so on. Tungsten trioxide has orthogonal, monoclinic, cubic, hexagonal and other crystal structure. Among them, hexagonal tungsten oxide causes many concern because of its special hexagonal passage; many metal ions can be embedded in this hexagonal channel, thereby forming a hexagonal tungsten bronze which exhibits potential application in anode material and rechargeable lithium-ion battery.
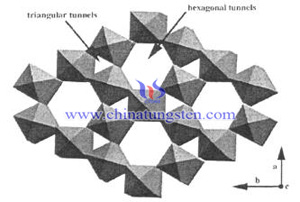
The crystal structure of the tungsten trioxide is ReO3 type, the result of A-site cation Absence of ABO3 perovskite structure, six oxygen atoms constituting the octahedron, W located therein and the neighbor WO6 octahedron links to form crystals by oxygen atom on the apical. Hexagonal tungsten trioxide has layered structure, and each layer octahedral connected by vertex to form a six-membered ring, in which the crystal axis direction will form a one-dimensional hexagonal channel. Further, the neighbor six-membered ring will form triangles which will also form a tripartite dimensional channel. Some scholars believe that the hexagonal and tripartite channels in hexagonal structure can accommodate cation, and has the chemical or adsorption interaction between each other, and each different cation can be substituted.
Hexagonal tungsten trioxide is a metastable crystalline phase, the preparation process is generally requiring moderate; it can generate by hydrothermal method from the raw materials of hydrochloric acid and sodium tungstate, with additives of potassium oxalate and potassium, respectively, hydrothermal synthesis method. Studies have shown that, at different temperatures, the various forms of tungsten trioxide crystal can be converted to each other, when the calcination temperature is 200°C, the product is orthorhombic tungsten trioxide; when the temperature is raised to 300°C, it begins to come out the peak of hexagonal phase tungsten trioxide; when the temperature reaches 450°C, hexagonal tungsten trioxide characteristic peaks disappears completely. Therefore, it can be concluded that hexagonal tungsten trioxide exists stably in interval of 200°C~450°C.
If you have any other question or inquiry of tungsten oxide, please feel free to contact us through the following methods:
Tel.: +86 592 5129696/86 592 5129595
Fax: +86 592 5129797



评论
发表评论